
As such, many authors have looked for clues, in the network structure, as to why the networks do not collapse ( Brose et al., 2006 Staniczenko et al., 2013 Borrelli, 2015 Gravel et al., 2016).
Since the early days of network ecology, ecological networks have been called “complex.” This sustained interest for the notion of complexity stems, in part, from the strong ties it has to stability ( Landi et al., 2018). Indeed, analysing ecological systems as networks highlighted how their structure ties into ecological properties and processes ( Proulx et al., 2005 Poulin, 2010), and there has been a subsequent explosion of measures that purport to capture elements of network structure, to be related to the ecology of the system they describe ( Delmas et al., 2018). We argue that SVD entropy provides a fundamentally more “correct” measure of network complexity and should be added to the toolkit of descriptors of ecological networks moving forward.Įcologists have turned to network theory because it offers a powerful mathematical formalism to embrace the complexity of ecological communities ( Bascompte and Jordano, 2007).

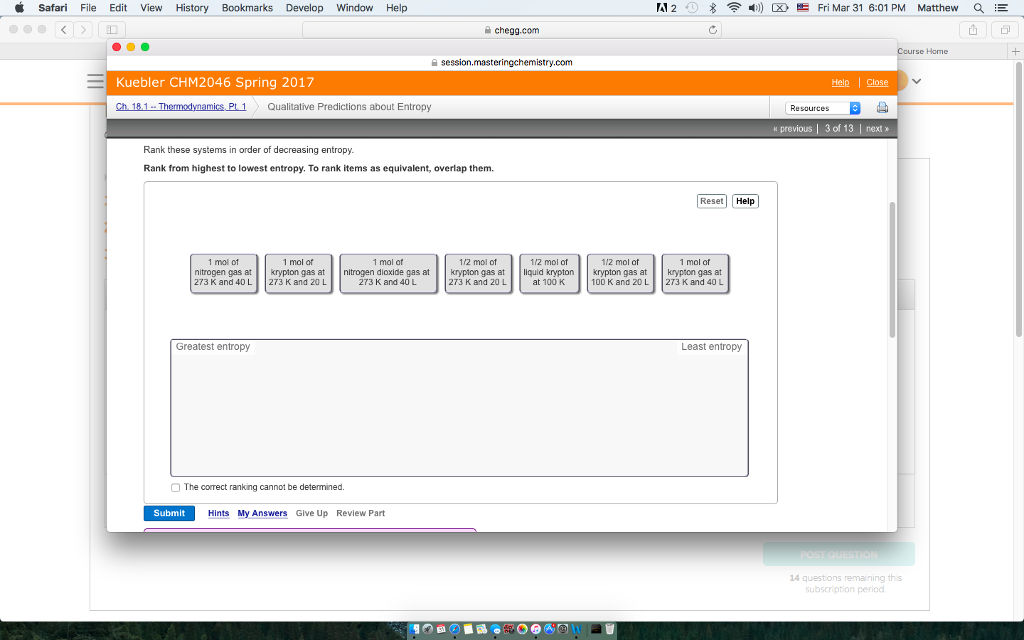
In addition, we find that SVD entropy relates to other structural measures of complexity (nestedness, connectance, and spectral radius), but does not inform about the resilience of a network when using simulated extinction cascades, which has previously been reported for structural measures of complexity. Pollination networks, in particular, are more complex when compared to other types of interactions. Using bipartite ecological networks, we find that they all show a very high, almost maximal, physical complexity. We present relative rank deficiency and SVD entropy as measures of “external” and “internal” complexity, respectively. These definitions ignore the notion of “physical complexity,” which can measure the amount of information contained in an ecological network, and how difficult it would be to compress. Primarily, complexity has been defined on the basis of the structural (or behavioural) complexity of the system. Quantifying the complexity of ecological networks has remained elusive. 3School of Mathematics and Statistics, University of Canterbury, Christchurch, New Zealand.2Québec Centre for Biodiversity Sciences, Montreal, QC, Canada.1Département de Sciences Biologiques, Université de Montréal, Montreal, QC, Canada.Dalla Riva 3 † and Timothée Poisot 1,2 * † You are so important.Tanya Strydom 1,2 †, Giulio V. The order of the entropy in the decreasing order is the lowest order. The liquid point of the gas is more important than the gas's entropy. The last mole of liquid is 20 liter and the entropy will decrease as well. Is there a mole? The half mole of the gas increases and the half mole of the gas decreases as the temperature gets cooler. It is in this 20 literoe 40 liter 1 mole of crypt with a greater entropy value.
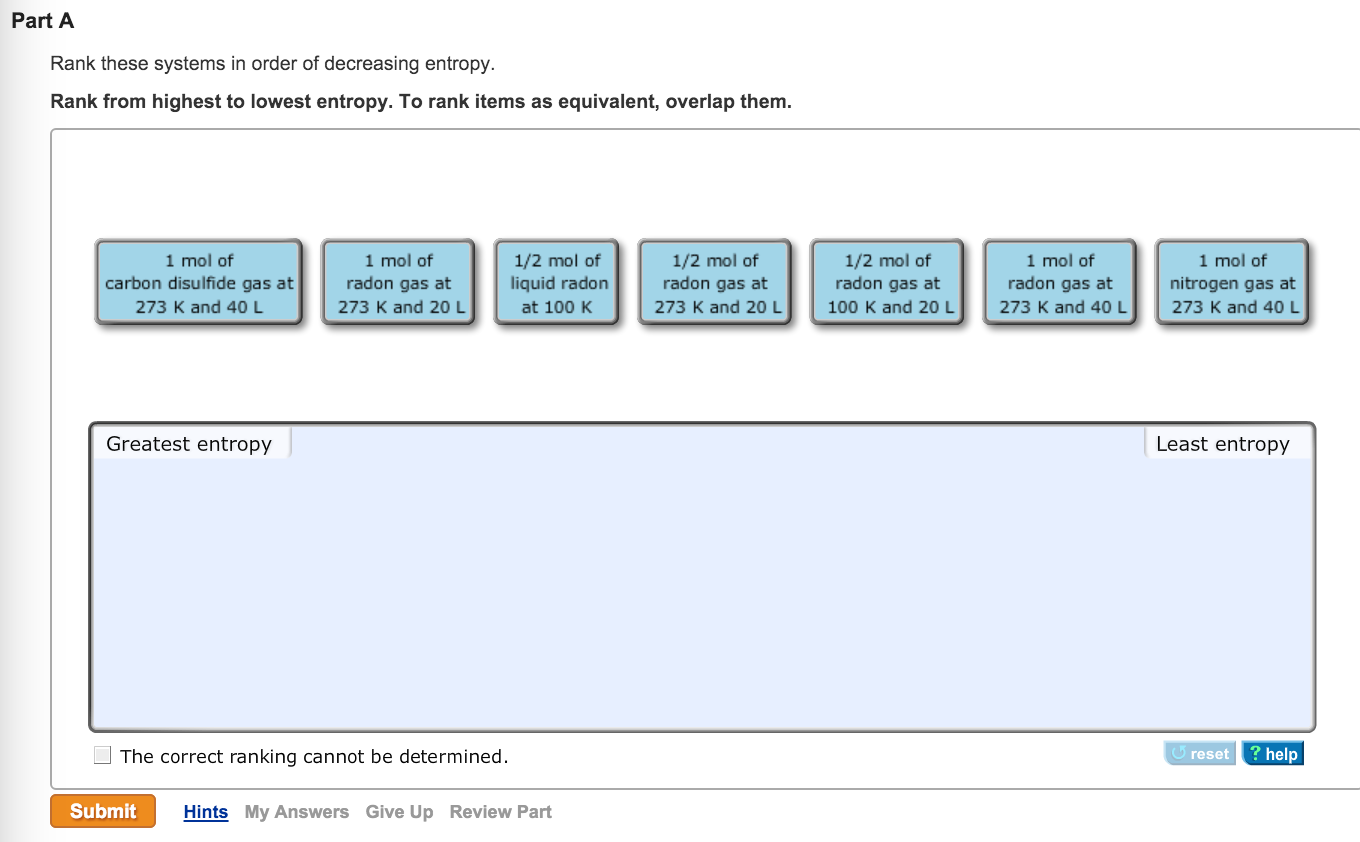
The 40 liter is the same as the 1 mole of the gas at 273kelvin and 20 liter. Next will be the mole of the gas at 273 and 40. There will be 1 mole of Florine gas at 273,kelvin and 40. We need to rearrange this in a different way.

The order of the entropy is that 1 mole of 1 mole of hydrogen peroxide gas at 273, kelvin and 40 litres will have the highest. If there is an increase in the volume and an increase in the temperature, the entropy increases. We can say that the moles of gas can be increased. The symbol of entropy is s, so if a guess is greater than the liquid, the guess's entropy is greater than that of gas. In the decreasing order, there are some terms that are related to the entropy. It is considered to be an extensive property of the matter that is expressed in terms of energy divided by temperature, and its unit is joule per kelvin. We know that the measure of the system's thermal energy per unit temperature is unavailable for doing useful work, so we are going to discuss about the measure of the system's thermal energy per unit temperature.


 0 kommentar(er)
0 kommentar(er)
